(National Book Foundation - New Book/New Syllabus Based on National Curriculum Pakistan 2023-2024 and Onwards)
(National Book Foundation - New Book/New Syllabus Based on National Curriculum Pakistan 2023-2024 and Onwards)
(Class 8 General Science Notes for 8th Centralized Exam 2023-2024 and onward, FDE, Islamabad)
(Class 8 General Science Notes for 8th Centralized Exam 2023-2024 and onward, FDE, Islamabad)
Click Here for Solved MCQs Unit-5
D. Structured questions
1. Following figure shows some elements in the Periodic Table
Ans: A and F
b) Which elements are present in the same period?
Ans: A and C, B and D, F and E
c) What is the group number of C?
Ans: IV-A
d) Which elements have same number of electrons in their outermost shell?
Ans: A and F
e) Which element is a noble gas?
Ans: VIII-A
f) Which element is alkaline earth metal?
Ans: II-A
g) In which group element E is present? VA [
Ans: O Group or VIII Group
h) Which element has two electrons in its outermost shell?
Ans: B
2. Element carbon (At. No. 6) is present in Group IV-A in the periodic table.
a) Why is carbon placed in Group IV A in the periodic table?
Ans: Because carbon has four electrons in its outermost shell.
b) How many electrons carbon needs to complete its valence shell?
Ans: Four electrons
c) How many single bonds carbon can make?
Ans: Four single bonds carbon can make.
3. Draw electronic structures of:
a) Oxygen (At. No. 8)
O = 2, 6
Period = 2
Group = VI-A
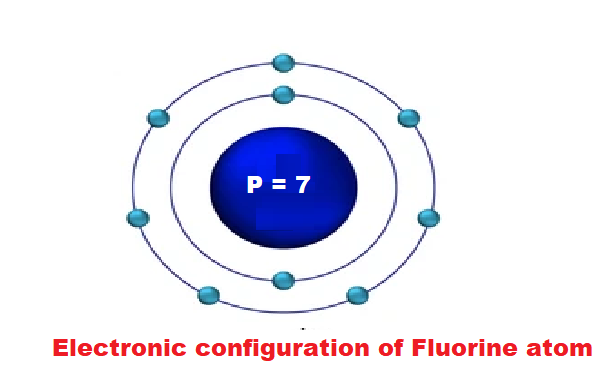
b) Fluorine (At. No. 9)
Write the difference in the electronic structure of these elements.
Ans: Oxygen, fluorine, and neon all belong to different groups on the periodic table, resulting in distinct electronic structures:
1. Oxygen (O): Atomic number 8. It has 8 electrons with 2 in the first energy level and 6 in the second energy level.
2. Fluorine (F): Atomic number 9. It has 9 electrons with 2 in the first energy level and 7 in the second energy level.
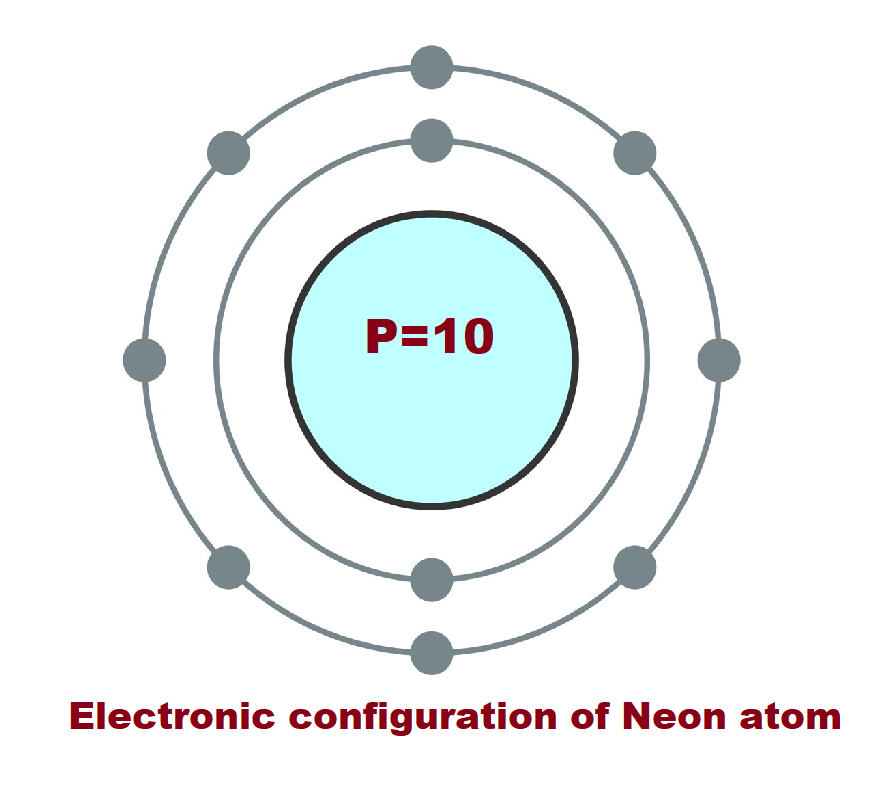
3. Neon (Ne): Atomic number 10. It has 10 electrons with 2 in the first energy level and 8 in the second energy level, making it a noble gas with a full outer electron shell.
E. Project work
1. Make an outline of the periodic table on a chart paper. Show the following elements on it some metals with brown colour, halogens with yellow colour, noble gases with red colour and some non-metals with blue colour.
2. Search and write the commercial uses of noble gases and alkali metals.
Ans: v
Noble gases, like helium and neon, are used in various commercial applications:
Helium: Used in balloons, airships, and as a coolant in nuclear reactors and MRI machines.
Neon: Used in neon signs, advertising displays, and as a component in high-voltage indicators.
Alkali metals, such as lithium and sodium, also have important commercial uses:
Lithium: Used in rechargeable lithium-ion batteries for electronic devices and electric vehicles, as well as in pharmaceuticals and ceramics.
Sodium: Used in the production of streetlights, fireworks, and certain types of glass, as well as in the chemical industry for various reactions and processes.
************************************
Class 8 General Science Notes (Main Page)
************************************
Shortcut Links For:
1. 5th Class All Subjects Notes
2. 8th Class All Subjects Notes
1. Website for School and College Level Physics
2. Website for School and College Level Mathematics
3. Website for Single National Curriculum Pakistan - All Subjects Notes
© 2023 & onwards Academic Skills and Knowledge (ASK)
Note: Write me in the comments box below for any query and also Share this information with your class-fellows and friends.



0 Comments
Note: Write me in the comments box below for any queries and also Share this information with your class-fellows and friends.